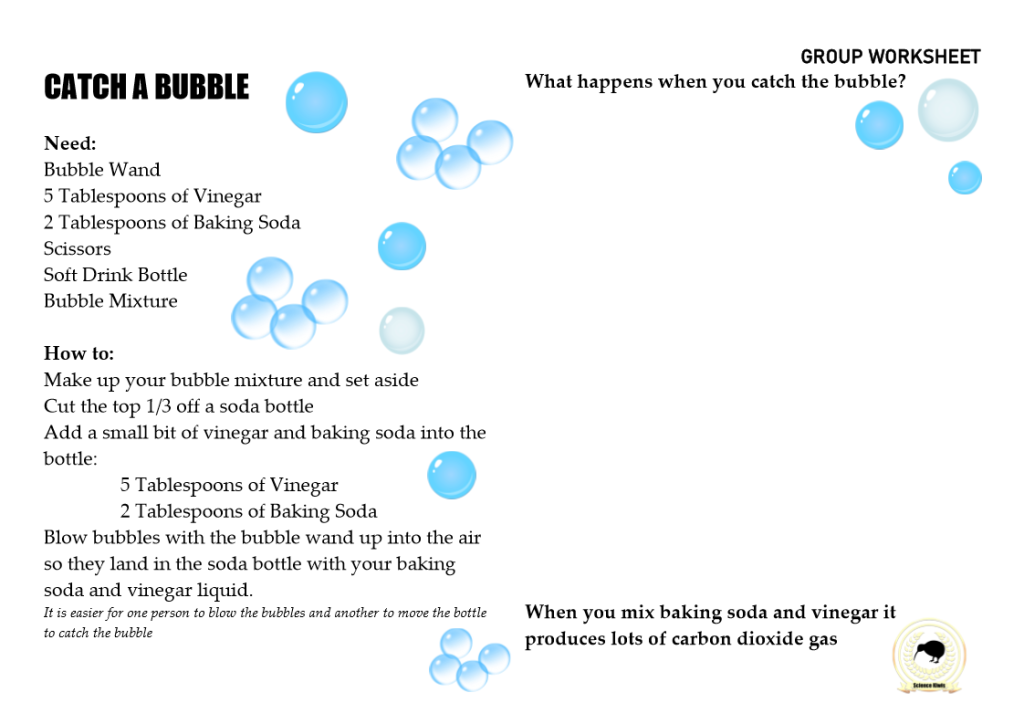
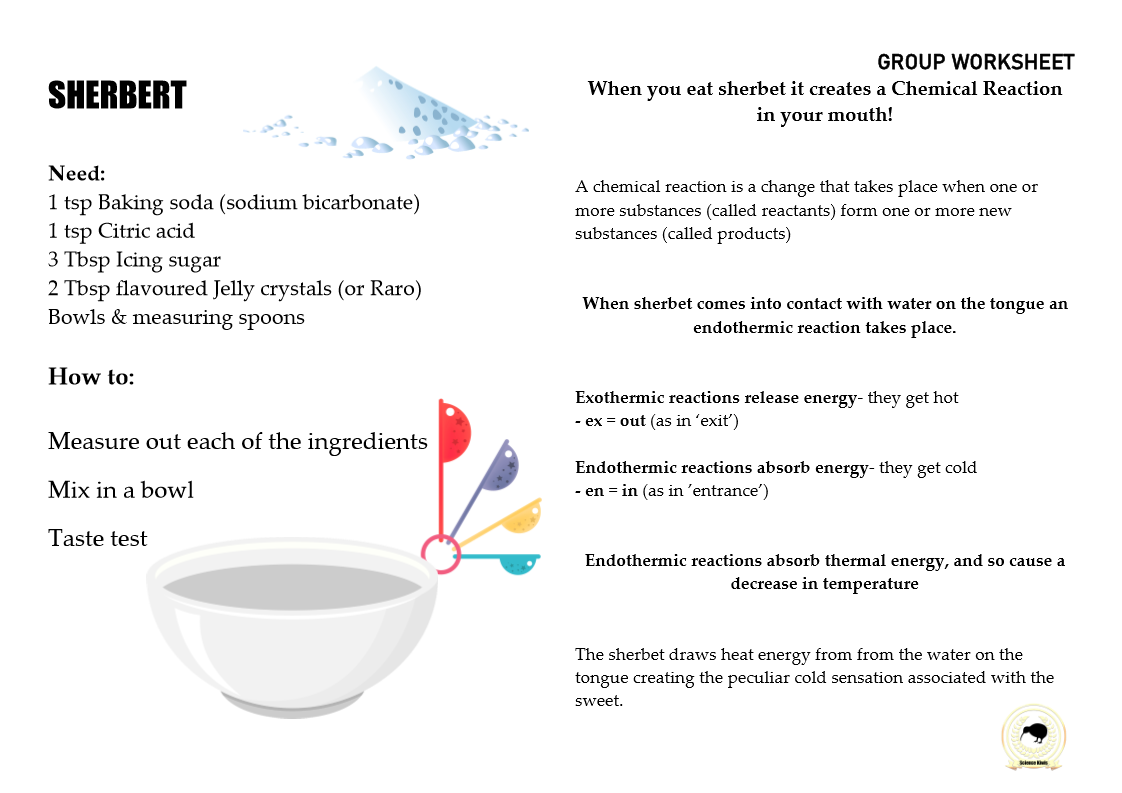
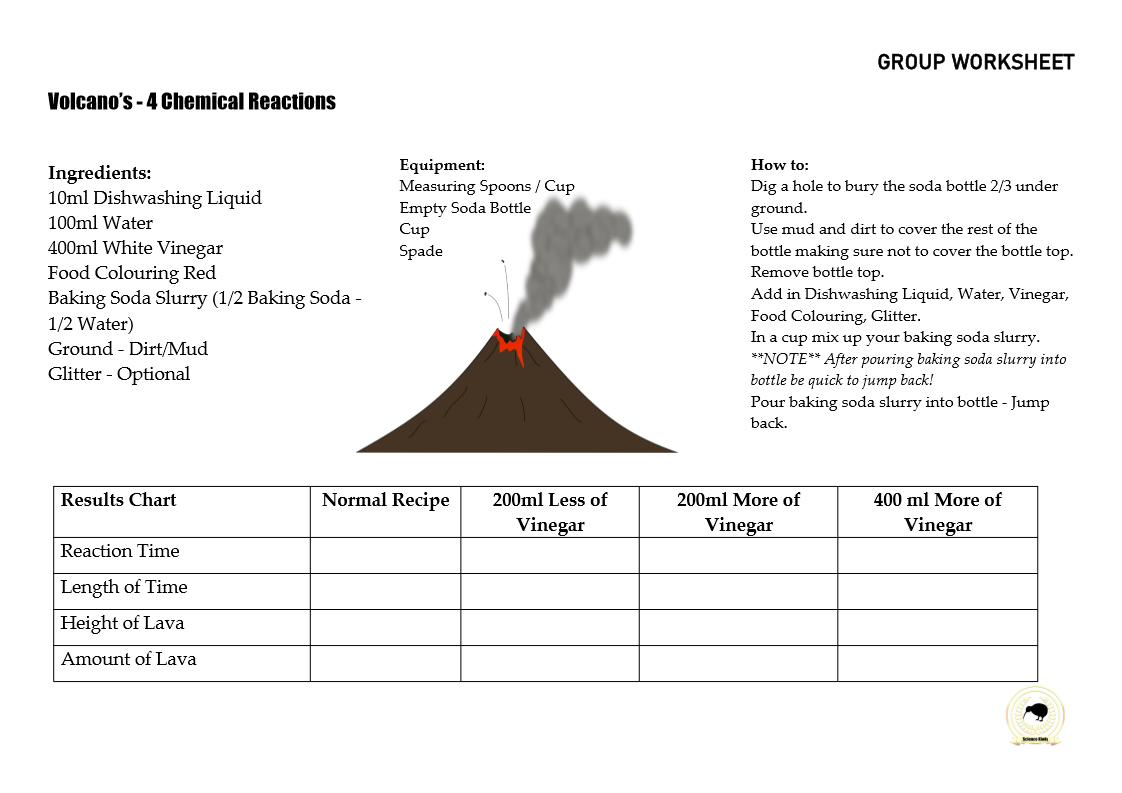
BUBBLES

…Bubbles…
Bubbles are a great topic to explore science concepts like solutions, gas, air, pressure, elasticity, soap films, liquids and surface tension.
What are Bubbles?
A bubble is an thin layer of soapy water that has enclosed a given volume of air.
The surface tension of the interface between liquid and gas creates a pressure difference.
For a bubble, the pressurized bubble of air is contained within a thin, elastic surface of liquid.
When the bubble bursts, the difference in pressure causes an audible pop.
How do bubbles form?
The formation of bubbles is because in the presence of a soap the water has reduced surface tension
Cohesion
Hydrogen atoms in a water molecule are attracted to oxygen atoms in another water molecule
Soap molecules help the water molecules be more stretchy and flexible as when they are added they decrease the force of attraction of the water molecules
The decrease of force is a decrease of the force of the surface tension
The soap when added and also when glycerin is added into a bubble mix reduces the evaporation of water in the mix so that the bubbles last longer when formed than if they where just water bubbles
Air bubbles blown into a glass of water are small and don’t last very long because of surface tension.
The water molecules surrounding the air bubbles attract each other, joining together.
Have you ever washed dishes?
The dish-washing liquid you add into the water bubbles up as you scrub the dishes
The dish-washing liquid is added because it makes the water soapy which is better at cleaning dirty dishes than just water.
Soapy water helps with washing things like oil, dirt, grease and foods away because by reducing the surface tension of the water it allows it to mix easier with the oils, dirt, grease which means that it comes off the dishes easily
Scientists refer to bubbles as minimal surface structures
This means that they always hold the gas or liquid inside of them with the least possible surface area.
Why are bubbles always round?
They are round because of surface tension.
A force called surface tension pulls the soap film tight, so that it always has the minimum surface area possible.
The wall of the bubble will automatically make the shape with the least surface area it can.
The air molecules trapped inside the bubble experience a force from the air molecules outside the bubble.
The air bubbles inside try to cluster together into a shape that minimizes their contact with the outside air.
This creates a spherical shape
How to blow bubbles
Make a bubble wand – check out the many ScienceKiwis instructions for bubble wands
Make up your bubble mixture – check out the many ScienceKiwis instructions for bubble recipes
Dip your bubble wand into a container of your bubble mixture
The bubble film will cling to all the edges of your bubble wand
After a few seconds gently lift your bubble wand out of the bubble mixture
Then blow air towards your bubble wand and then a bubble should come out of your bubble wand
DOCUMENTS :
Click on the file name to open and download
IMAGES :
To download / save / print – Simply right click and save image.
Images:



Documents:
Bubbling Bubbles Bubble Mixture